INTRODUCTION:
Despite improvements in the treatment and outcomes of patients with acute myeloid leukemia (AML), in nearly 10-20% of the cases, a morphological remission (CR) after administrating induction regimens is not achieved. In other cases, and despite initial CR, early disease relapse may occur, with only a minority of patients durably benefiting from salvage regimens. Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative strategy for patients with relapsed / refractory (R/R) AML. However, the prognostic of these patients after allo-HCT remains dismal, with reported survival rates ranging from 20% to 35% in different publications.
Allo-HCT using peripheral blood and sequential-based conditioning regimens has been the transplant platform used at our institution for R/R AML during the last decade. During the study period the use of post-transplant cyclophosphamide (PTCY) combined with tacrolimus (TK) has been implemented on this allo-HCT setting.
This study evaluates the results obtained from a single institution obtained from using sequential allo-HCT for patients with R/R AML, and the effectiveness of having implemented the use of PTCY in this type of transplant. Very few studies have evaluated the safety of including PTCY-based in sequential-based conditioning allo-HCTs.
METHODS:
This study includes the 21 patients with R/R AML who underwent allo-HCT with sequential-based conditioning regimens at our institution between 2011 and 2022. Retrospective data were collected during June 2023.
Sequential regimens combined fludarabine (150 mg/m2), cytarabine (2 g/m2 x 5 days), and idarubicin (12 mg/m2 x 3 days) chemotherapy with low doses of Bu (n=3), melphalan (n=17), or treosulfan (n=1). Six adults transplanted after November 2016 received PTCY (50mg/kg/24h) on day +3 and +4 followed by TK from day +5 to day +90 for GvHD prevention.
RESULTS:
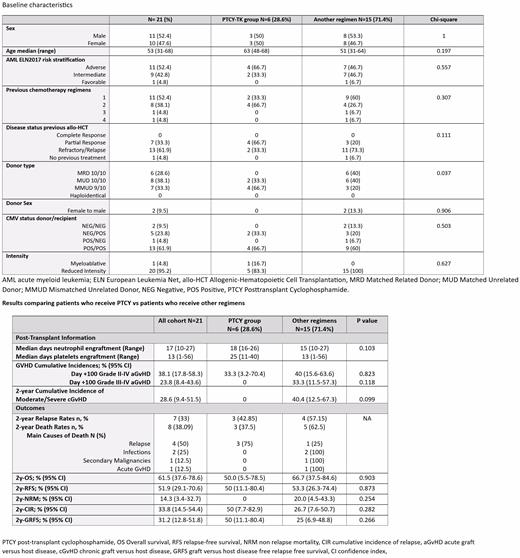
The main baseline characteristics of the study cohort and according to the GVHD prophylaxis are shown in Table 1 . The median age was 53 years (range 31-68), 11 (52%) patients were males, and all patients had R/R AML after having received at least one line of induction chemotherapy with curative intent. Fourteen (66.7%) patients received grafts from HLA-matched donors, and 7 (33.3%) from 9/10 HLA mismatched unrelated donors (MMUD). Baseline characteristics between patients who received PTCY and those who did not were balanced, except for the proportion of allo-HCT performed from MMUD which was higher in the PTCY group (66.7% vs. 20.0% p=0.03).
Post-transplant results are shown in Table 2. All patients engrafted (100%). Patients who received PTCY experienced similar median of days to neutrophil engraftment (18 vs. 15 days, P=0.103) and a longer median of time to platelet engraftment (25 vs. 13 days, P=0.046). The day +100 cumulative incidences (CI) of grade II-IV and III-IV aGVHD, and 2-year CI of moderate/severe cGVHD were 33.3%, 0% and 0% for patients who received PTCY-TK, and 40% (p=0.823), 33% (p=0.118), and 40.4% (p=0.099) for those who did not.
During the first 2-years after allo-HCT, 8 (38%) out of 21 patients died and 7 (21%) relapsed. The main causes of death were relapse (n=4, 19%) and infection (n=2, 9.5%), and the 2-year OS, PFS, NRM and CIR were 61.5%, 51.9%, 14.3%, and 33.3%.
As shown in Table 2 patients who received PTCY had comparable rates of OS (2-y: 50.0% vs. 66.7%, p=0.903) and RFS (2-y: 50.0% vs. 53.3%, p=0.873) and those who did not. In addition, and although no patient who received PTCY died secondary to transplant-related toxicity (2-y 0 vs. 20%, p=0.254), a non-significant trend to higher cumulative incidence of relapse (2-y: 50.0% vs. 26.7%, p=0.282) was observed in this group of patients. Lastly, a non-significant trend to higher GRFS was documented in patients who received PTCY (2-y: 50.0% and 25.0%, p=0.266).
CONCLUSIONS:
At our institution, 50% of the patients with R/R AML going thought allo-HCT performed using sequential-based conditioning regimens survived after two years. The implementation of PTCY as part of the GVHD prophylaxis results decreased rates clinically relevant GVHD and NRM, resulting on a trend to better GRFS. Although the results provided by this analysis suggest that using PTCY in this allo-HCT is safe and effective, caution is still recommended considering the trend to higher relapse rates observed among these patients and the limited sample size.
Disclosures
Díaz-Beyá:Bristol Myers Squibb: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Jazz Pharma: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Martínez-Roca:BMS: Honoraria, Other: travel grants; Abbvie: Honoraria, Other: travel grants; Gilead: Other: Travel grants; Janssen: Other: travel grants; Takeda: Honoraria, Other: travel grants; Roche: Honoraria, Other: travel grants; Kite: Honoraria, Other: travel grants. Jimenez-Vicente:Pfizer: Other: Travel Grants; Abbvie: Other: Speaker, Travel Grants. Rosiñol:Amgen: Other: Honoraria for lectures; Sanofi: Other: Honoraria for lectures; Takeda: Other: Honoraria for lectures; GlaxoSmithKline: Other: Honoraria for lectures; Bristol Myers Squibb/Celgene: Other: Honoraria for lectures; Janssen: Other: Honoraria for lectures. Esteve:Gilead: Consultancy; Pfizer: Research Funding; Abbvie: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding; Kronos Bio: Research Funding; Astellas: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal